What does carbon dioxide have to do with making bread?
What causes staff of life dough to ascent?
15 January 2021 | Gary Tucker, Fellow
A not bad deal is written almost the way that yeast produces carbon dioxide to leaven breadstuff dough during proof. Yet, there are other gases such as ethanol and steam that are vital during the oven stages of bread making. These gases tend to be forgotten only without them information technology would not exist possible to bake bread of the quality we expect. Their role increases in importance as the bread reaches the stop of the bake fourth dimension when its construction becomes more susceptible to plummet. In this article I'll talk over the different gases that are essential in helping breadstuff dough to ascent during baking.
There are many ways of making staff of life from the basic ingredients of flour, h2o, yeast and salt, from labour-intensive artisan staff of life to high-throughput sandwich breadstuff. However, there are 3 aspects that are critically important to any type of bread making, and it all starts in the mixer:
- The mixing action entrains numerous air bubbling into the dough that can be inflated during proof and baking.
- Dough evolution takes place during the mixing process to convert the flour proteins into a gluten matrix that has the elasticity to allow the bubbles to expand in afterward stages.
- Gases are generated by yeast activeness and/or chemical leavening agents to expand the bubbles and create the desired aerated bread structure.
The role of gases in expanding the bubbles during proving and baking is fascinating but usually focuses on yeast converting sugars to carbon dioxide (CO2). Yeast metabolism in dough is complex and involves a short aerobic fermentation stage where oxygen from the bubbling is used upwardly followed by a much lengthier anaerobic stage. The details of this are explained subsequently. The mechanisms help to explain why the dough volume initially shrinks, why there is a lag before the dough gradually increases in volume and how oven spring takes place. Nonetheless, as mentioned in that location are other leavening gases that play a vital office, particularly during baking, and without them the staff of life is certain to plummet in the oven. This article highlights the office of the other gases together with the mechanisms by which those gases piece of work to inflate the bubbles.
Background enquiry
Among the many research papers on bread dough leavening at that place a couple that are worthy of mention. These introduced the concept that gases other than carbon dioxide played a key role in expanding gas bubbles in dough. Moore and Hoseney (1985) calculated the carbon dioxide volume from expansion of the gas bubbles and ended that carbon dioxide solitary did not explain the increase in volume from dough to breadstuff during baking. They thought that ethanol was pregnant, especially effectually 70°C when its vaporisation charge per unit increased rapidly. A farther publication on this subject was by Bloksma (1990) who compared the Moore and Hoseney calculations with his own on fractional pressures of gases during baking. Bloksma included carbon dioxide, ethanol and water in his own calculations of thermal expansion. He concluded that water (in the grade of steam) contributed more than half of the oven spring volume, with ethanol and carbon dioxide responsible for much of the balance. These calculations were made upward to 70°C because this was the stage when Bloksma considered that oven spring ended and further volume increase was negligible.
Gases involved in bread making
As mentioned previously, during breadstuff blistering in that location are several gases that contribute to the leavening of breadstuff dough. Carbon dioxide is the main gas associated with yeast leavened breadstuff, withal, other gases that play a part are ethanol, nitrogen and steam. At that place is too a minor contribution from low molecular weight volatile compounds formed during fermentation. The chief gases and their roles are described in Table 1.
| Gas | Clarification |
|---|---|
| Carbon dioxide (COii) | Generated by yeast during aerobic and anaerobic fermentation of glucose. Amylases convert starches in the flour to maltose, which is further converted to glucose by maltase from the yeast. Anaerobic fermentation is the ascendant effect. |
| Ethanol (C2H5OH) | Generated by yeast during anaerobic fermentation of glucose. Molar quantities of ethanol are the same as for CO2 during anaerobic fermentation. Ethanol boils at 78.iv°C so its influence on dough expansion is significant equally the dough temperature approaches seventy°C. |
| Water (H2O) | Approximately 40-60g is lost from a 900g dough piece (to brand an 800g lidded loaf) during blistering. Much of this will be water which will turn to steam late in the oven. |
| Nitrogen (Northward2) | Nitrogen is left in the bubbles when oxygen is used past both yeast and ascorbic acrid. Nitrogen expands as information technology increases in temperature. Dissolved gases (oxygen and nitrogen) will also come out of solution as the dough liquid increases in temperature. |
| Other volatiles | Volatile molecules are also generated from fermentation, such as carboxylic acids, aldehydes, ketones and alcohols. |
Table 1: Gases released from bread during baking
Carbon dioxide (CO2)
Carbon dioxide generation takes place during ii stages of fermentation because yeast can metabolise both aerobically and anaerobically. Aerobic fermentation is the start pathway and volition continue until all the oxygen is used up and the atmospheric condition in the dough become anaerobic. In that location is competition with ascorbic acrid for oxygen, added to increase gluten oxidation during mixing, and this limits the extent of aerobic fermentation. The yeast first metabolises glucose and oxygen to carbon dioxide and water, as in Equation 1. Glucose is generated past enzymic pathways from the starch in the flour to maltose and then to glucose. Oxygen comes from the air in the bubbles entrained during mixing.
C6H12O6 + 6O2 → 6CO2 + 6H2O Equation 1
Having used the available oxygen, subsequent fermentation during proof takes place with the dough in an anaerobic condition. Yeast obtains the oxygen needed direct from the glucose, according to Equation ii. This is by far the near dominant phase in yeast fermentation. Equal molar quantities of carbon dioxide and ethanol are produced from glucose breakdown, which are significant, and discussed later.
CviH12Osix → 2COtwo + 2C2H5OH Equation 2
Information technology is important to note that the carbon dioxide produced from either fermentation mode does not go straight into the gas bubbling. At proof temperatures of 32-38°C, carbon dioxide is soluble in water to a level of about 1.0 g/kg. It first dissolves into the aqueous phase that surrounds the yeast cells and continues to dissolve until the liquid becomes saturated. During the aerobic stage, oxygen is used from the air bubbles and this reduces the pressure inside each bubble. They first compress by nigh 20% equally they at present only contain nitrogen. Just when the water is saturated with carbon dioxide can it enter the bubbles to inflate them. Carbon dioxide can also dissolve in organic solvents such as the vegetable oil used in bread making. This helps in a small-scale way to increase the quantity of dissolved carbon dioxide in the dough.
Ethanol (CiiH5OH)
Equation 2 shows that ethanol and carbon dioxide are produced in equal molar quantities during anaerobic fermentation. Ethanol is readily soluble in water and organic solvents, to the extent that all the ethanol produced during fermentation dissolves into the liquid phases surrounding the gas bubbles. Information technology boils at 78.4°C and is likely to have left from the bread soon after its humid point of 78.4°C is reached. The rate of ethanol evaporation is probable to be at its highest when the internal core temperature is in a higher place 70°C.
The office of ethanol in dough behaviour is circuitous and is a subject for farther investigation. Ethanol is known equally a universal solvent because it allows both polar and non-polar compounds to dissolve. Information technology therefore increases the solubility of carbon dioxide so that more tin deliquesce and be available to release from solution as the dough temperature rises during baking. This contributes to the sudden oven spring that happens early in the baking process. Ethanol too increases the solubility of the gliadin wheat poly peptide fractions, which may have an touch on the rheological behaviour of dough because the gliadins are idea to confer extensibility to dough. Another property of ethanol is the part it plays in softening bread nibble.
Steam (Htwo0)
Water boils at 100°C and and so will be released subsequently into the blistering process than ethanol (BP 78.4°C) or carbon dioxide (BP -56.6°C). By the time steam is released into the bubbles information technology is likely that the construction of a baked production will have increased in 'viscosity' essentially because of starch gelatinisation that takes place above lx°C. However, the thin gas bubble walls will still incorporate a lot of h2o and are very soft at this stage. Without the increase in pressure from ethanol and steam it is likely that the delicate bubbles will collapse.
Nitrogen (N2)
Air contains approximately xx% oxygen and 80% nitrogen. Oxygen is used up past the yeast during its aerobic stage and is replaced in the gas bubbling by carbon dioxide once the liquid surrounding the gas bubbling is saturated. Nitrogen is unaffected by yeast fermentation and will expand as the bread temperature rises. The contribution of nitrogen to bubble expansion is less meaning than with the gases that come up out of solution. Nitrogen expansion is uniform with temperature compared with the instant volume increment when a gas comes out of solution.
Each gas exerts its pressure influence at a different stage in the oven, and without these gases working in series information technology is not possible to create a crumb structure based on such a fragile network of bubbling.
When are the gases released?
This is another interesting question. Soluble gases such as carbon dioxide and ethanol dissolve into the aqueous and organic liquids surrounding the bubbles. The extent of their solubility depends on pressure and temperature. As the dough temperature increases, the gases are forced out of solution and inflate the gas bubbles. If a bubble breaks and coalesces with an next bubble, the gas is retained within the larger bubble and is not lost. Just a small corporeality of gas is lost from the pinnacle surface of the dough. Experiments have shown that this is non a significant quantity and merely occurs towards the end of proof and more then with less well developed doughs.
Oven leap takes place quite early into the blistering process. This is when a sudden increase in force per unit area occurs within the bubbles, which causes the dough to strength upwardly apace. Expansion slows downwardly when the sides and top surfaces dry, set up hard and they resist the upwardly forces. Further volume increase is express and, with pan breadstuff, causes a break in the side crusts towards the upper edges. This force per unit area increase is caused mostly by carbon dioxide coming out of solution simply in that location is some contribution from nitrogen expansion and ethanol. Oven spring is usually completed in the showtime third of the bake.
The internal network of bubbles in the bread nibble remains delicate for the duration of the bake. Without a positive pressure to maintain them, the bubble walls are soft enough and then they tin hands plummet. The bubble pressure is showtime maintained by carbon dioxide because it boils at the lowest temperature (-56.6°C), followed past ethanol (78.iv°C) and finally by water (100°C). Nitrogen expansion takes identify gradually as the dough temperature increases throughout. Each gas exerts its pressure influence at a different stage in the oven, and without these gases working in series it is not possible to create a crumb structure based on such a delicate network of bubbles. Figure 1 illustrates the temperature rise in an 800g loaf of sandwich bread during blistering, with the approximate regions in which the gases have their influence on maintaining the bubble pressure. Figure 2 is a micro CT (computerised tomography) image taken for a 2cm cube from a white sandwich breadstuff that shows the fine gas bubble structure. It would not exist possible to create such fine and delicate crumb structures without a series of gases to maintain the internal gas pressures.
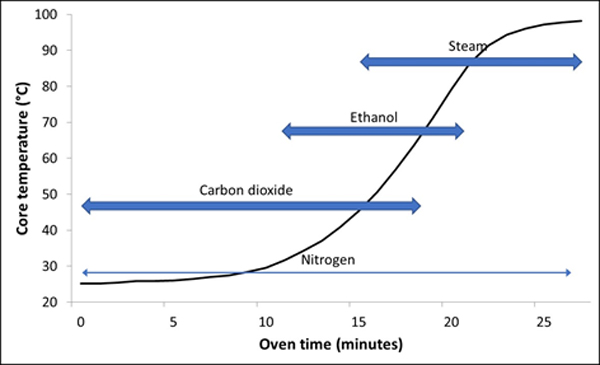
Fig 1: Time of influence of the different gases during baking of white sandwich bread

Fig two: Micro CT prototype of the porous structure in a 2cm cube cut from of a white lidded loaf
Foam to sponge conversion
In that location is one terminal change in the bread crumb structure that is essential to avoid plummet of a loaf later baking. This is specially important with staff of life broiled in lidded pans where crust thickness and strength are much less than with open broiled crusty products. Every single gas bubble must break to let the hot gases out. If the intact gas bubbles cool, the result volition exist a loss in pressure and enough volume reduction to cause the side walls to collapse inwards. This change – where the bubbling break to allow the escape of their gases - is known equally the foam to sponge conversion and is idea to happen just a few minutes before the bread exits the oven. The result is effectively ane very complex gas bubble made from all the interconnected smaller gas bubbles.
Towards the end of the oven process, and just before the foam to sponge conversion, the gas bubbles contain a mixture of gases including nitrogen, steam, carbon dioxide, ethanol and some volatile organic compounds. It is likely that steam is mostly responsible for breaking each of the gas bubbles in bread; converting the construction from a airtight foam to an open up sponge. When water changes into a gas it increases in volume substantially. The later stages in blistering are when the bread is hot enough for steam generation to occur rapidly.
This conversion is an essential part of the blistering process and enables the gases to escape to atmosphere and exist replaced by cooler air. Without this escape pathway each bubble suffers a massive plummet in pressure and volume as the gases cool.
Summary
The function of carbon dioxide, ethanol, nitrogen and steam is fascinating during mixing, proof and baking. It would not be possible to broil staff of life with a fine network of gas bubbles without each of these gases working together to maintain the force per unit area within the bubbles. Carbon dioxide is responsible for the volume increment in dough during proof and for much of the oven spring that happens early into the bake. Ethanol and steam are vital to maintain force per unit area inside the delicate bubbles towards the later on stages of blistering. Finally, and after the starches take gelatinised and started to set, steam creates force per unit area that breaks each of the chimera walls to release the gases into the oven temper. Air rushes back into the newly created single interconnected bubble to brand the bread construction more than resilient to collapse. Past agreement these different mechanisms during mixing, proof and baking it is possible to pattern recipes and processes, and to solve blistering problems more easily.

Nearly Gary Tucker
Gary Tucker is a Young man at Campden BRI and has worked for the company since 1989. He studied Chemical Applied science at Loughborough University and is a chartered chemical engineer.
Read more than...
Sympathise broiled products ameliorate with our baking courses
Understand broiled products improve with our baking courses from baking nuts to biscuits and across. Take a look at the full range of loftier quality training that'due south delivered by our experts to develop and abound your workforce's skills and talents.
Source: https://www.campdenbri.co.uk/blogs/bread-dough-rise-causes.php
0 Response to "What does carbon dioxide have to do with making bread?"
Post a Comment